Scientists have establish a way to create propylene using a light-activated catalyst. The energy-efficient process could lower emissions to synthesis this broadly utilized commodity chemical and aid industrial decarbonization.
Propylene is the basis for the polymer polypropylene which is used in everything from clothing fibres to packaging and household goods. Annually, more than 150 million tonnes is manufactured and the mixed synthesis of ethylene and propylene creates about 900 million tonnes of carbon dioxide. Chemists at Northwestern University have now synthesized a photoactive catalyst which can make propylene directly by propane dehydrogenation (PDH) – a technique requiring milder situations than conventional steam cracking. Non-oxidative PDH has been gaining recognition as it yields the favored product exclusively however consists of challenges such as higher operational temperatures. ‘The chemical industry uses lots of energy [that] comes from the combustion of fossil fuels,’ comments senior author Dayne Swearer. ‘This study was absolutely seeking to leverage the idea utilizing of power to power chemical processes.’
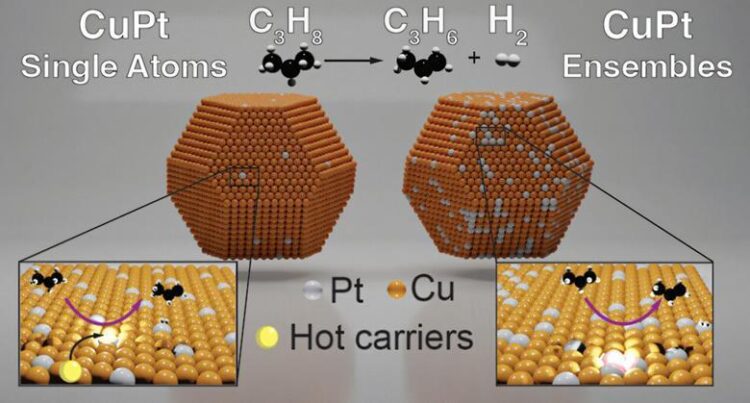
The team nanoengineered an alloy of copper doped with platinum to harness the light from LEDs or the solar to convert propane to propylene. Copper is a well-established optical plasmonic material and platinum is highly effective at C-H bond activation, so the team decided to fine tune the material by changing the concentration of the bimetallic species. ‘People had looked at this particular material in the past, but no one’s really shined light on it to see how that changes and manipulated these reaction pathways,’ stated Swearer. Once they had created the catalyst, ‘that’s when we start doing our catalytic testing … shine the laser on and off whilst we’ve a certain temperature factor… The idea is to reduce the temperature point at which we operate,’ explains lead creator Emma-Rose Newmeyer.
They discovered that the atomically dispersed platinum sites had been vital for selectivity and reactivity – making the alloy much less highly-priced – and engineering of the atomic sites may want to enhance the catalyst over those that included more platinum atoms. ‘These experiments shed light on the fact that this was a photochemical method instead of a photothermal process,’ comments Newmeyer, emphasizing that there is underlying chemistry taking place on the surface. The single platinum atoms act as reactive hotspots which facilitate C-H bond activation, a important step in converting propane to propylene. Experiments revealed enhanced reactivity on the platinum sites, allowing reactions at temperatures 50°C decrease than changed into feasible thermally. ‘This is an thrilling locating which can potentially encourage new reactor standards to decease the overall temperature of operations whilst increasing conversionper pass,’ comment Jimmy Faria Albanese, catalytic materials researcher at the University of Twente, Netherlands.
The team now plans to discover numerous other instructions. ‘We want to look other natural gas species which are tougher to activate and notice what we will convert there,’ stated Newmeyer, even as Swearer provides that understanding that light modifies chemical pathways will be important in direct conversion of methane to propylene and hydrogen on an industrial scale.
Although Faria Albanese is optimistic about the prospects of non-oxidative dehydrogenation of alkanes, he’s wary of the scalability of the brand new photocatalyst. ‘The activity appears to decrease substantially over short periods of time. This could likely restrict the application of this system in a real industrial scale,’ he adds.