A new catalyst lets in selective manufacturing of hydrogen and acetic acid from ethanol, and not using a direct carbon dioxide emissions
The researchers after the work agree with that the reaction ought to not compete with current commercial methods for generating the chemicals.
Around one hundred million tonnes of hydrogen per year are utilize in industry for applications together with ammonia production and elimination of sulfur from petroleum. At this time, the massive majority comes from fossil fuels, especially through steam improving of methane – that is itself strongly endothermic. Decreasing the carbon footprint of the chemical industry, consequently, needs a greener resource of hydrogen, and a few chemical engineers are trying to bioethanol. The essential routes by means of which ethanol can generate hydrogen are partial oxidation, in which the ethanol reacts with oxygen, and full steam reforming, in which it reacts with water. In both cases the carbon and oxygen go to carbon dioxide.
In the brand new work, researchers at Peking University in Beijing, collectively with colleagues some other place in China and Graham Hutchings in Cardiff, UK, show selective partial steam improving. This rather produces acetic acid – which is required for producing other chemicals consisting of acetates – because of the oxidised byproduct. ‘This reaction isn’t definitely new,’ says physical chemist Ding Ma, who headed the research. ‘But in the past people have not been able to attain high selectivity in the direction of acetic acid, or they have utilize the base in order that they go to the acid salt in place of the (acetic) acid itself.’
The researchers used a catalyst aid on molybdenum carbide, which Ma’s group and others have already proven to have unheard of ability to generate water molecules at low temperatures. To activate ethanol, the researchers brought platinum and iridium. ‘It turns into platinum is extra energetic
(than iridium) for ethanol improving… Moreover, platinum tends to combination into small clusters,’ says Ma.
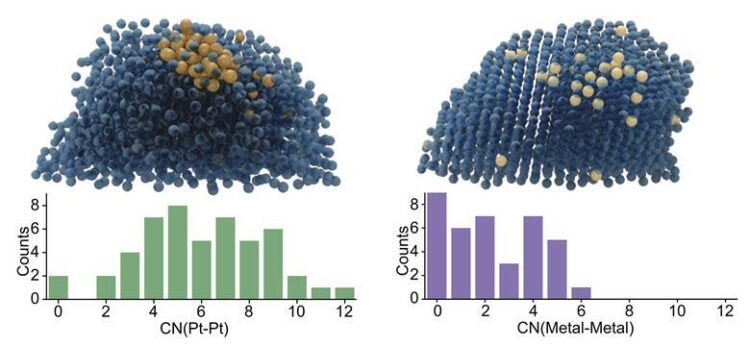
Metal–metal bonds in those clusters can activate and break the ethanol’s carbon–carbon bonds, main to the generation of carbon dioxide or methane. Iridium, however, reacts very strongly with the aid, dispersing atomically and helping to block nucleation sites for platinum clusters.
Spectroscopic imaging confirmed that, as a result, both metals were almost atomically dispersed at the floor. This intended that most effective one of the ethanol substrate’s carbon centres was activated, and so the response produced almost no chain breakage.
The researchers found that their reaction changed into less susceptible to side reactions than both partial oxidation or steam reforming. At 270°C, they carried out an acetic acid selectivity of 84.5%. A preparatory techno-financial evaluation leads the researchers to finish that the response will be aggressive with current processes for generating the chemicals, although Ma says that ‘in future if one could decrease the amount of iridium or probably replace the whole catalyst with non-noble metals it would be much better.’
Fabio Ribeiro, who co-directs Purdue University’s engineering take action on main energy-transition enhance and pathways to sustainability, believes the take a look at may be scientifically thrilling – mainly if it offers novel insights into reaction mechanisms or catalysis. However, Ribeiro has reservations approximately the method’s commercial viability.
‘At current charges, the overall cost of the hydrogen and acetic acid produced is less than the price of the ethanol and method water,’ he explains. ‘How can you are making a profit in case your very last product are worth much less than your starting materials?’
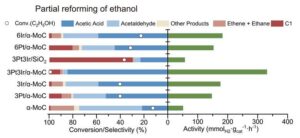
The platinum–iridium catalyst outperformed others in terms of both activity and selectivity for the acetic acid reaction product
Ribeiro, who also directs the US National Science Foundation’s Center for Innovative and Strategic Transformation of Alkane Resources, stated that bioethanol manufacturing commonly calls for fertiliser, which itself normally relies on ammonia, suggesting that the feedstock is not likely to be carbon-impartial. Furthermore, despite the fact that all the global ethanol were dedicated to this response, it would only effective to meet a small fraction of global hydrogen needs at the same time as manufacturing an extra of acetic acid a long way past current needs. ‘When it comes to simply green hydrogen, the great path is water electrolysis powered by way of renewable energy, that’s already cost-aggressive with steam methane reforming in some areas,’ he says.
Responding to the concerns about the economic possibility of the procedure, Ma points out that the global prices for both ethanol and acetic acid can vary substantially on an annual and local foundation, ‘making the profitability of any future version of this procedure highly sensitive to change’.
‘For instance, over the last ten years, cost in China and the United States have shown substantial one-of-a-kind trends and fluctuation,’ he notes. ‘In america, ethanol is much cheaper than acetic acid due to the availability of grain-derived ethanol. Conversely, in China, coal-derived methanol is considerable, ensuing in lower acetic acid charges.’
Ma additionally admits that water electrolysis is the most feasible approach for making hydrogen, but says that the energy performance of that procedure is low. ‘Our method indicates that there’s scope for alternative methods to make green hydrogen together with other chemicals, which is also suitable for allotted small-scale produce,’ he adds. ‘Besides, this procedure might additionally make contributions to defossilising current acetic acid manufacturing route. Indeed, the future will now not be “a one size fits all” for chemical production and our new method, which is as yet in its infancy, shows there may be a way forward.’